Clinical Trial Template

Investigator initiated clinical research data and safety monitoring guidelines and policies clinical study templates and forms nih and other federal guidelinespolicies for clinical research.
Clinical trial template. Mock study section planning guide planning guide oncore clinical trial management system ctms new user request form required fields and definitions regulatory support and protocol templates find templates and forms on the institutional review boards irb website including. The toolbox contains templates sample forms and information materials to assist clinical investigators in the development and conduct of high quality clinical research studies. This template provides a general format applicable to all clinical trials that are evaluating study productsinterventions. Nih and fda release protocol template for phase 2 and 3 indide clinical trials not od 17 064.
Preparing to apply for a u01 clinical trial registering with clinicaltrialsgov patient research registries clinical trial policies guidelines and templates. Welcome to global health trials tools and templates library. Please note that this page has been updated for 2015 following a quality check and review of the templates and many new ones have been added. The nccih clinical research toolbox provides a web based information repository for investigators and staff involved in nccih funded clinical research.
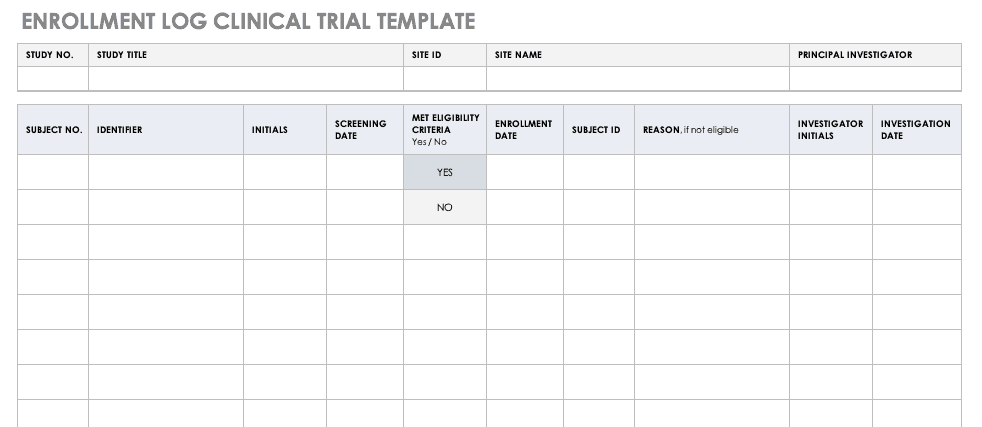
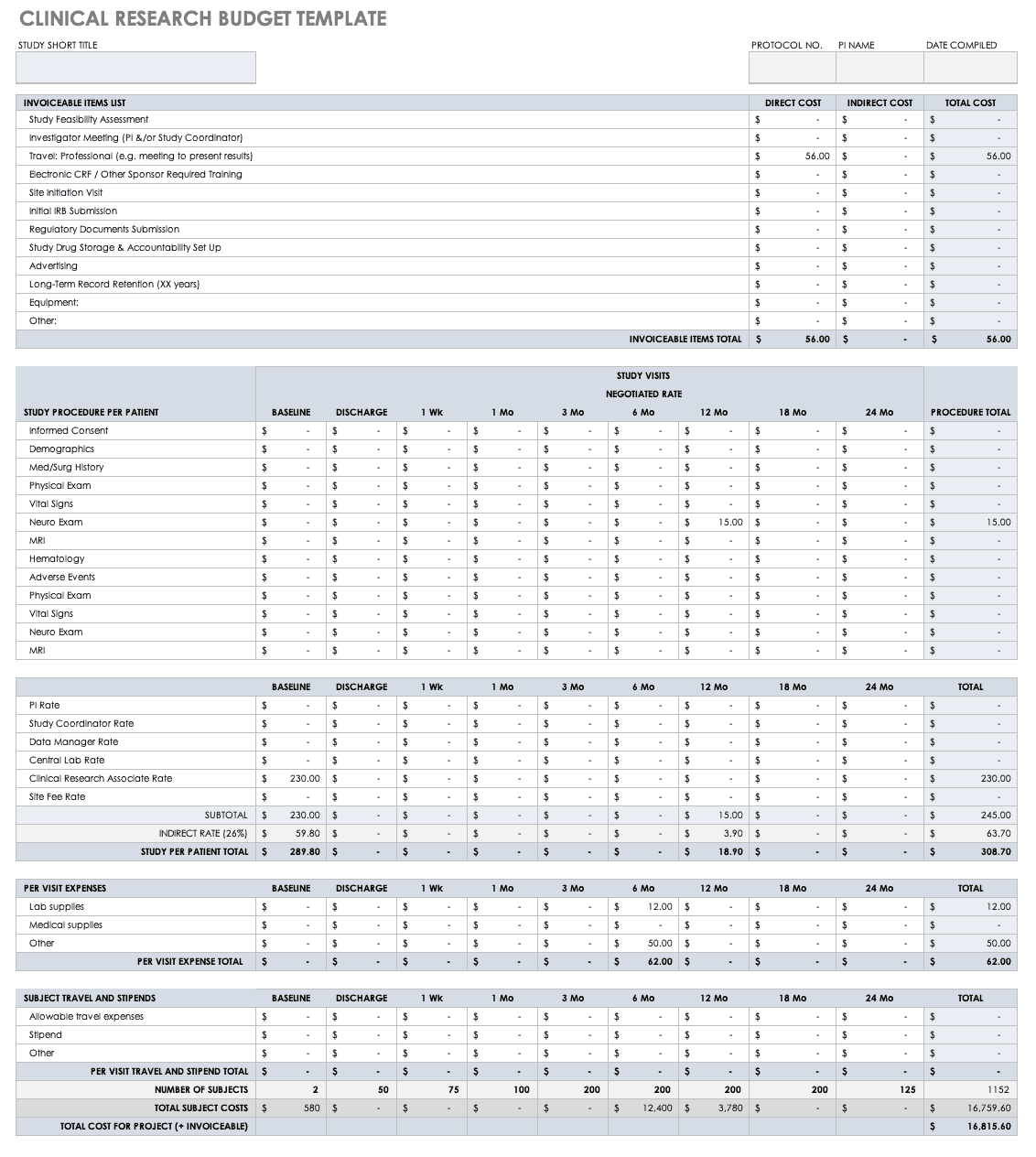
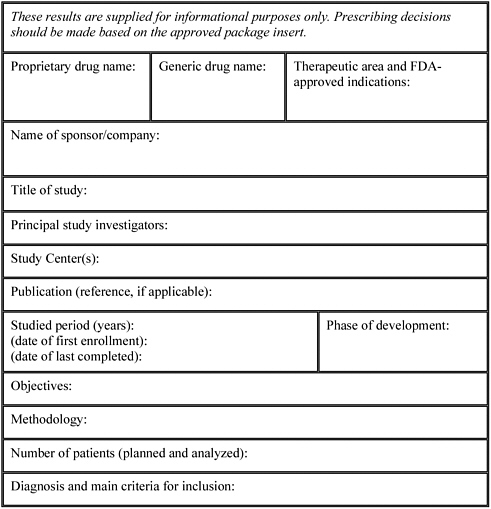
Trial template is a trial graphics system that installs directly into powerpoint. The clinical trial template has site lists of libraries for clinical trial protocols protocol documents announcements calendars issues tasks and document discussions. Samples forms and worksheets contents adverse eventintercurrent illness log advertisement sampleapproved budgeting by activity worksheet budgeting by position worksheet case report form concomitant medications condentiality letter or nondisclosure agreement contact worksheet for sponsor and vendors contract or clinical trial agreement. Include each template section in the protocol as applicable.
The first type of trials are phase 2 and 3 clinical trial protocols that require a food and drug administration fda investigational new drug ind or investigational device exemption ide application. Nih funding opportunities and notices in the nih guide for grants and contracts. Protocol templates consent templates irb submission documents. These can be further customized with different versions of sharepoint.
Trial template contains an extensive library of drag and drop animations timelines medical slides document call outs and other litigation graphics that have successfully been used by top trial attorneys.