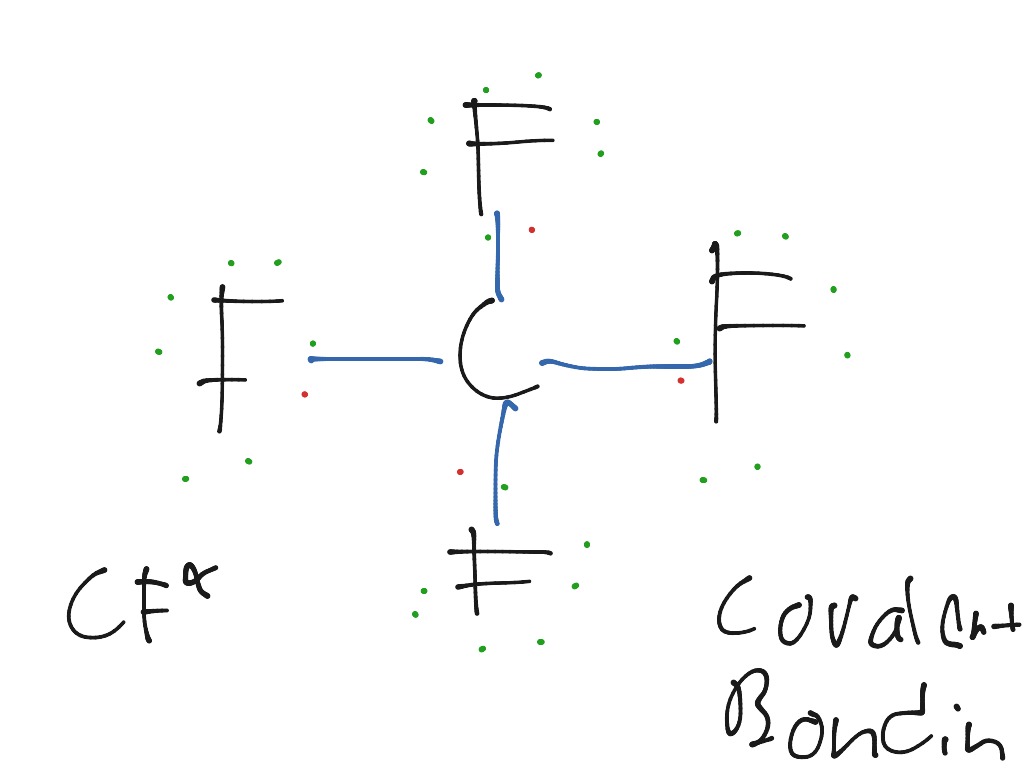
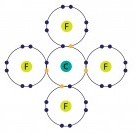
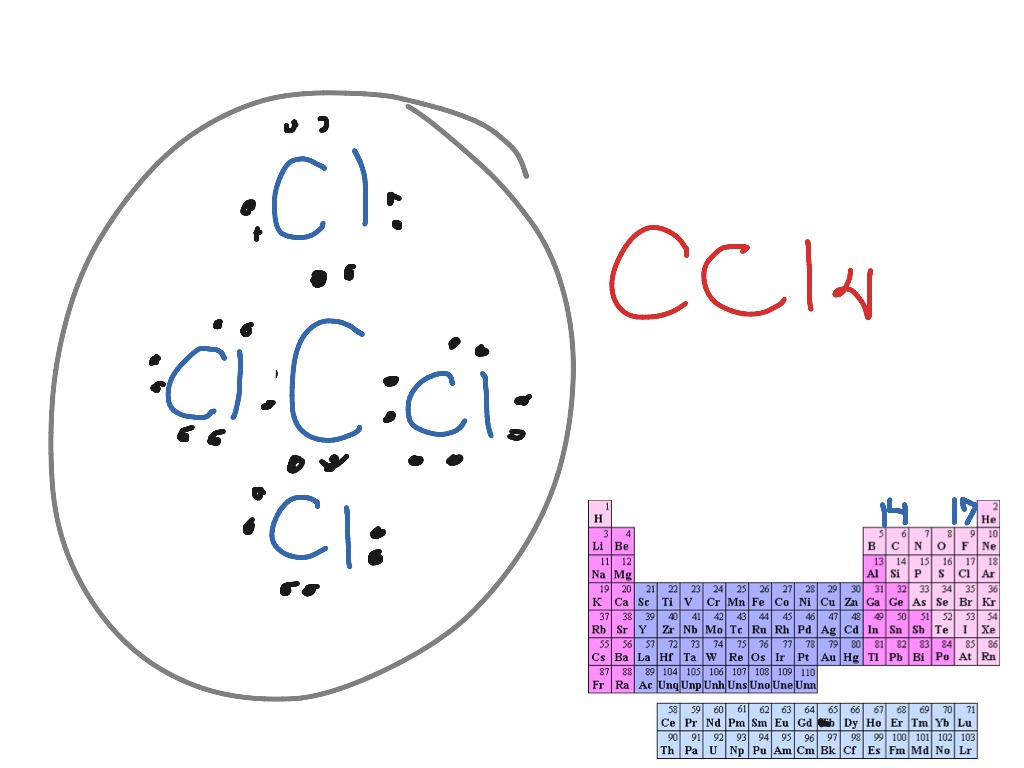
Dot And Cross Diagram Of Cf4

Pls i need it for school project.
Dot and cross diagram of cf4. For the cf 2 cl 2 lewis structure there are a total of 32 valence electrons available. Carbon fluoride dot and cross diagram posted on june 2 2019 by admin try drawing dot cross diagrams to show the following covalent bonds hydrogen fluorine carbon dioxide tetrafluoride this is how the ionic bond forms in aluminium fluoride alf3 youtube draw a dot and cross diagram for of carbon dot cross diagram c2 topic 3 4 16. Draw li atom with one dot. Cf4 1 carbon x fluorine.
For dot and cross diagrams you first need to identify which element will be cross and which will be dot. Can anyone draw a dot and cross diagram for carbon tetrafluoride cf4 and hydrogen sulphide h2s. So 4 dots around it. C atom has 4 electrons in outer orbital.
Draw f atom with three electron pairs and one electron as cross. This incomplete dot and cross diagram shows only the bonding pairs of. Either ionic or covalent. The difference is that you have both cl and f.
Each 4 dots should be paired with one cross at a time of four f atoms. 5 so it forms three covalent bonds. When a current flows through a conductor it produces a magnetic field shown in the above diagram as the green circular arrows surrounding the wi. There are three shared spaces between the circles so add a dot and cross to each one.
Lewis structures also known as lewis dot diagrams lewis dot formulas lewis dot structures electron dot structures or lewis electron dot structures leds are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. As that way you have to do others. The lewis structure for cf 2 cl 2 is similar to cf 4 or ccl 4. After you figureout how many bonds there is between which atoms.
Since they are in the same group on the periodic table they each have the same number of electrons 7. Since carbon is the least electronegative element it goes in the center of the lewis structure in the lewis structure of cf4 there are a total of 32 valence electrons. Drawing dot and cross diagram for carbon tetrafluoride and hydrogen sulphide. Then you need to know how many electrons there are on each atoms outershell picture how a single atom would look in your mind.
The odd electron cross should pair with the dot of li atom.