Fda Certification Requirements

Any representation of fda registration number on product label or labeling which implies fda certification or fda approval of a facility or product is misleading and may cause misbranding of the product.
Fda certification requirements. However they still are considered artificial colors and when used in cosmetics or other fda regulated products they must comply with the identity specifications uses. Each manufacturer shall establish procedures for identifying training needs and ensure. A each person engaged in the manufacture processing packing or holding of a drug product shall have education training and experience or any combination thereof to enable that person to perform the assigned functions. Fda approval of color additives.
This linking using the information in form fda 3674. These color additives are obtained primarily from mineral plant or animal sources. Employees involved in manufacturing warehousing or packaging each employee that has a role in the production or storage of a drug needs to have sufficient education training experience or any possible combinations. Fda approval is required for color additives used in food drugs cosmetics and some medical devices.
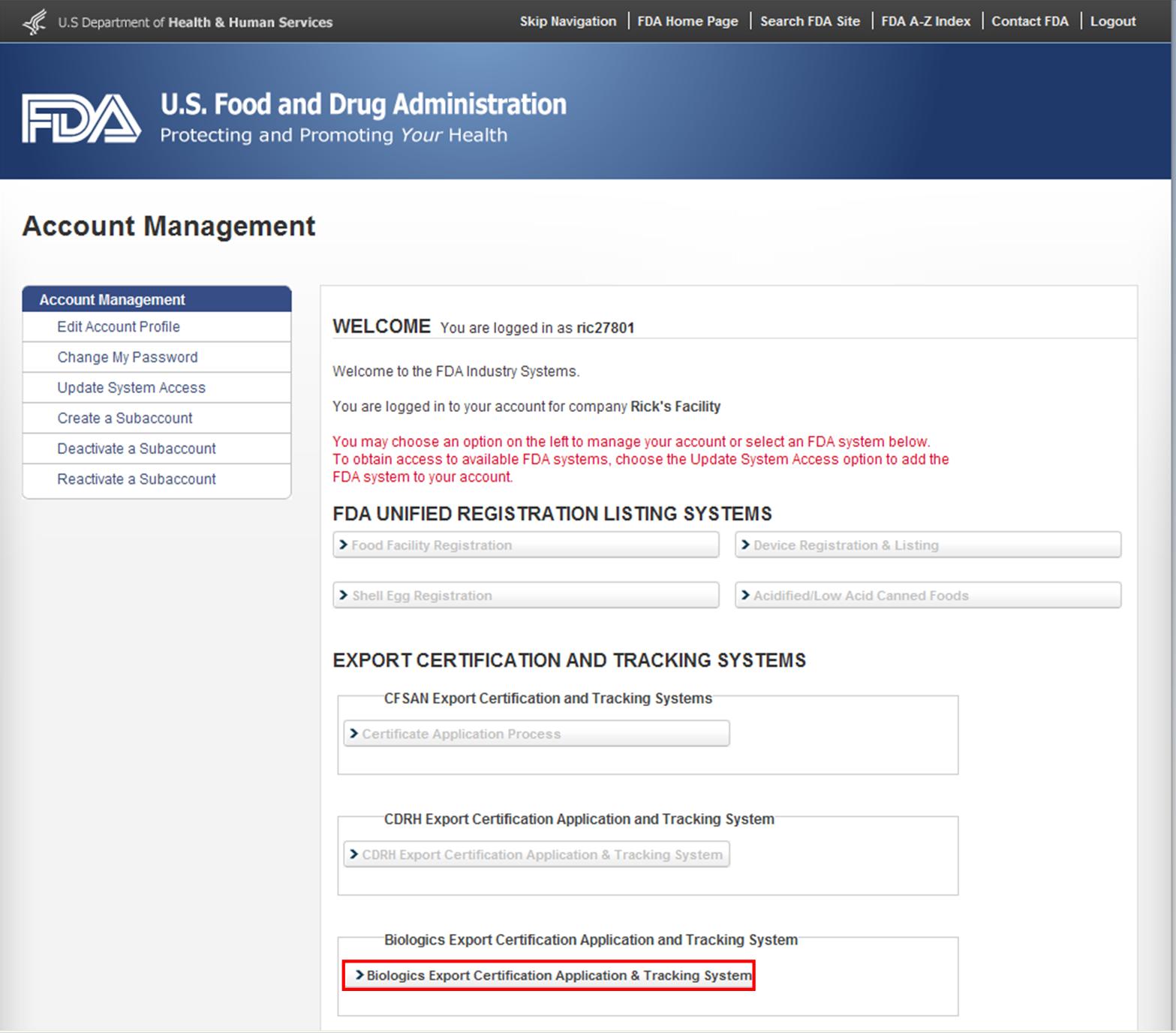
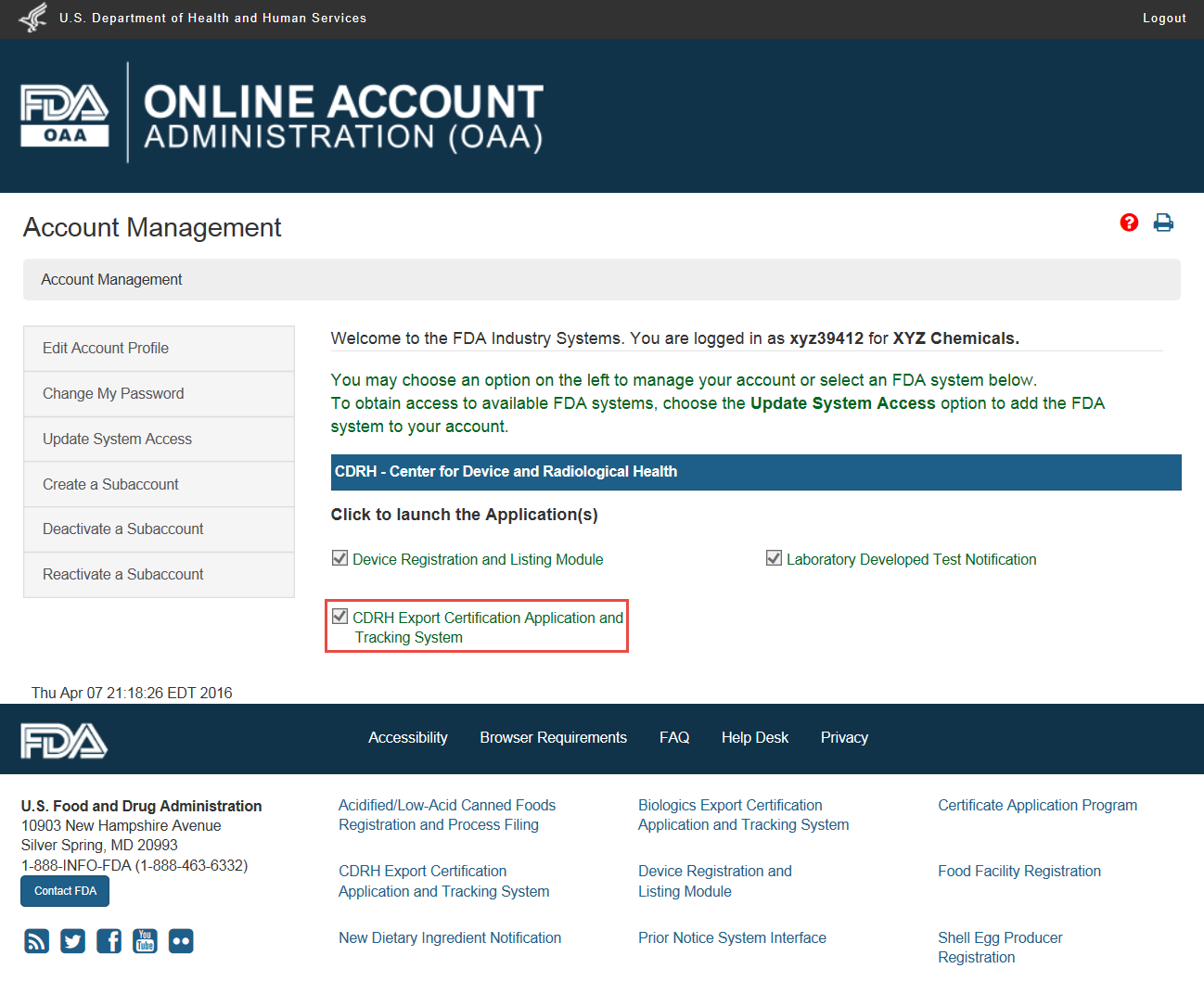
Fda registration or fda registration number does not denote fda certification or approval of your facility or products. Color additives may only be used in compliance with their approved uses specifications and restrictions. They are not subject to batch certification requirements. Training must occur for all types of regulated activities from internal quality management procedurespolicies to cfr requirements.
Find education and resources related to fdas regulatory product quality and safety responsibilities. The agency expects companies to establish comprehensive procedures. Fda learning portal for students academia and industry. Fdas cgmp requirements for drugs are the requirements for the methods to be used in and the facilities or controls to be used for the manufacture processing packing or holding of a drug.
The information provided in the certification form helps us assist nih in linking information posted on fdas website regarding certain fda regulatory actions to specific applicable clinical trials included in the registry and results databases. The fda requires medical device and drug manufacturers to train their employees. Educational resources and training opportunities for healthcare professionals industry consumers and academia.