Iso 13485 Templates

Imsxpress iso 13485 template documentation is part of imsxpress iso 13485 software.
Iso 13485 templates. If you hold an iso 134852016 certification then you are recognized as being able to manufacture medical devices that meet or exceed industry standards. Streamline your team effort with a single tool for managing documents projects and communication. By downloading your free preview of the iso 13485 toolkit youll experience firsthand the quality and extensive knowledge that go into our toolkits. Charles bradshaw be careful what you use when building up your qms system.
Iso 134852016 is the international standard for quality management systems qms used by organizations involved in the manufacturing servicing distribution and disposal of medical devices. Tools conformio is a smart online compliance tool implement and maintain iso 13485 gdpr iso 27001 iso 9001 iso 14001 or other iso standards in your company with ease. The medical device qms templates are used by our consultants in the field and are full of practical guidance and how to instructions. The expanding internet means more.
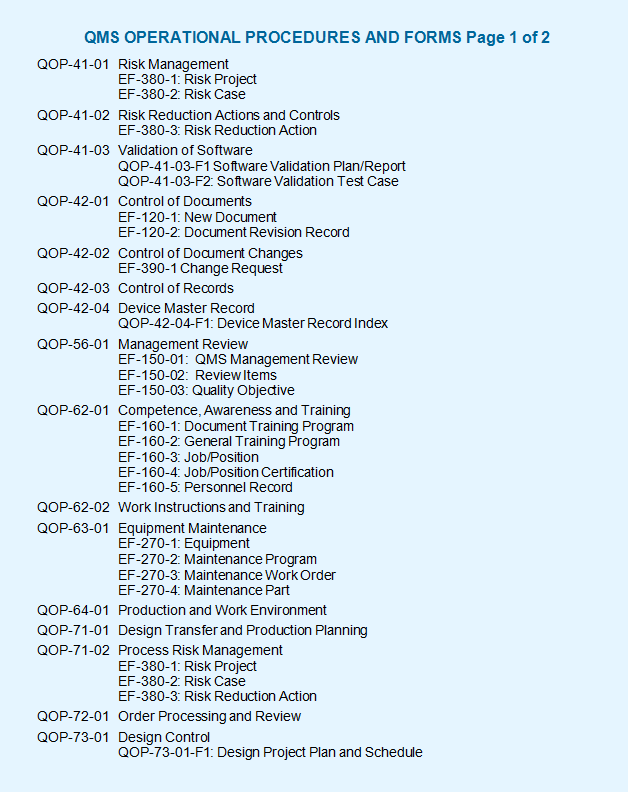
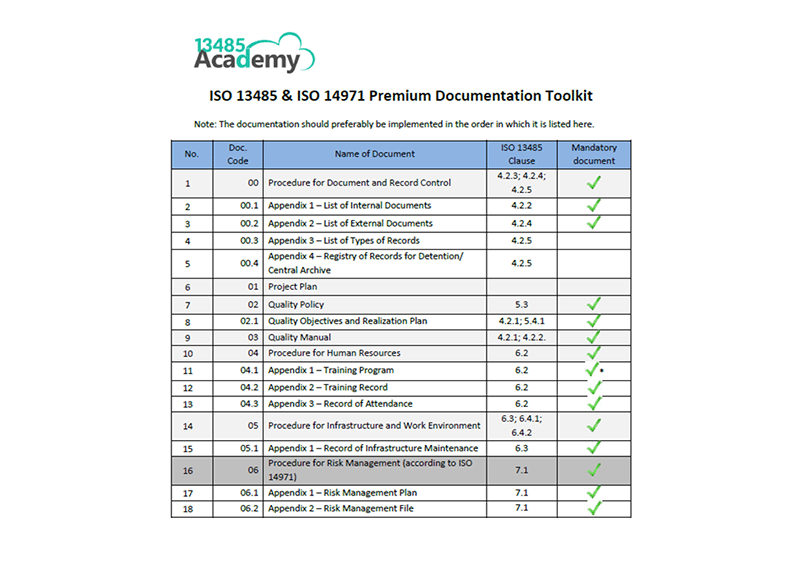
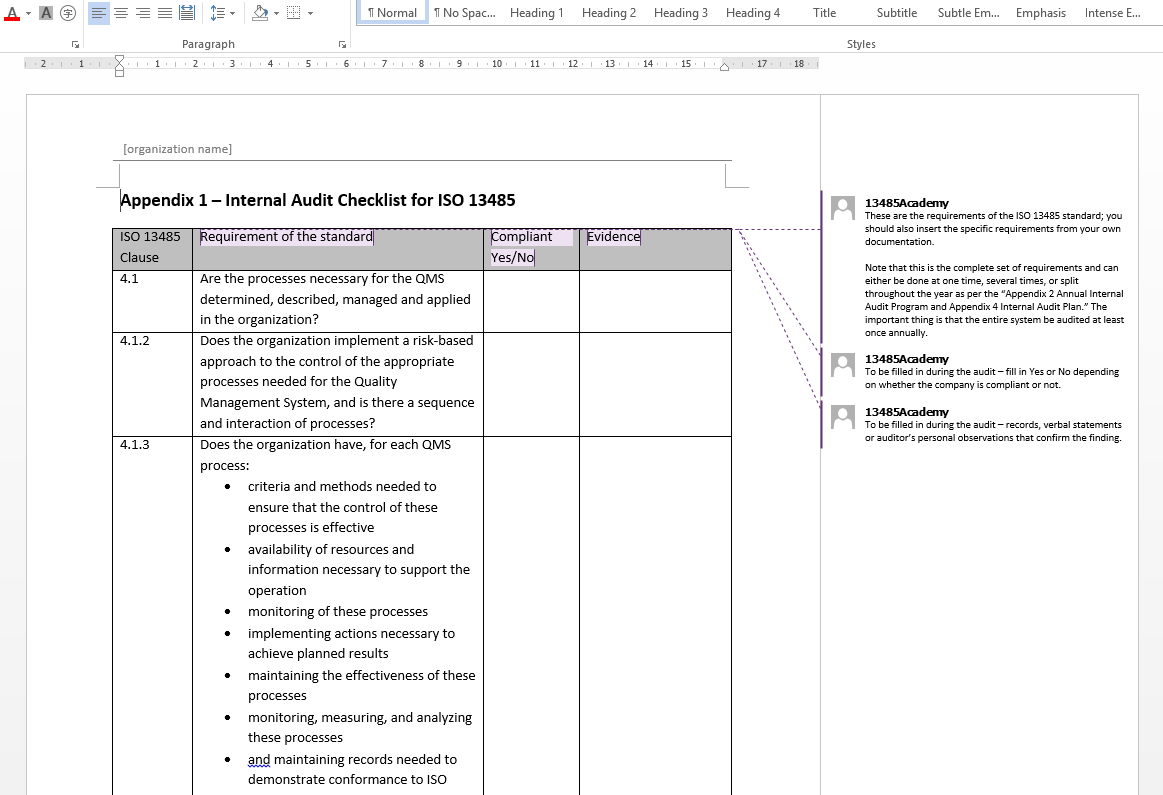
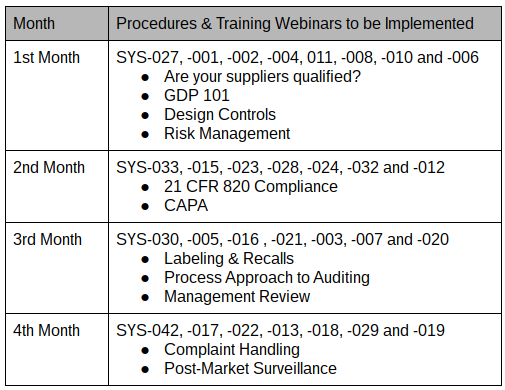
The iso 13485 documentation is a very easy process if it is developed with the basic knowledge of iso 13485 documents and medical devices in quality management system qms. The iso 13485 documentation kit include are iso 13485 quality manual procedures for quality management system exhibits and sops sample format and forms maintaining record process flow chart as well as iso 13485 audit checklist templates written in english. Iso 134852016 flowcharts created in ms visioc and smartdrawc. These flowchart templates can be exported to ms wordc.
This document combines all the previous advice that i provided. Built in microsoft word for easy editing these medical device qms templates are the quick and easy way to build a quality management system qms compliant with the iso 13485 standard or qsr 820 regulations. You can find hundreds of both expensive and free templates for iso 13485 offered by various consultants companies and. Iso 13485 manual and procedures template first of all on this package i prepared a procedure writing template in word.
Theyre designed to make your iso implementation fast and easy. The pros and cons of the 4 best iso 13485 gap analysis templates. The temptation of iso 13485 template kits. Iso 134852016 is an international standard for the quality management system qms of organizations involved in the manufacturing distribution servicing and disposal of medical devices.
The template documentation covers both iso 134852003 and fda qsr 21 cfr part 820 requirements under one quality system and is thus ideally suited for companies that must comply with both the us fda and international regulations. Organizations with iso 134852016 certification are recognized to produce medical devices that are at par with industry standards.