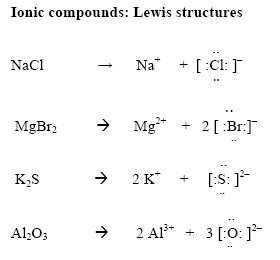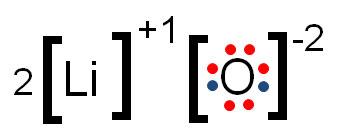Lewi Dot Diagram Ionic Bond

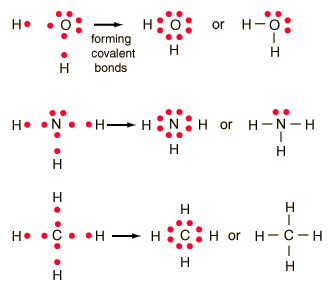
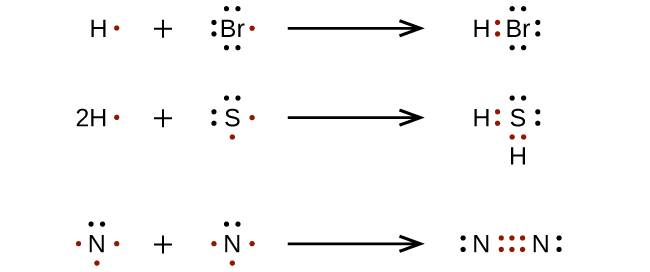
Single bonds are represented by a pair of dots or one line between atoms.
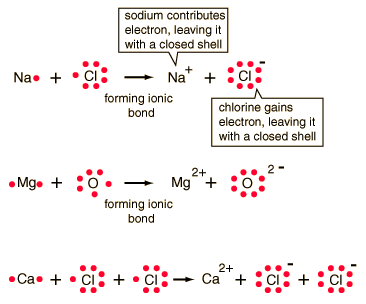
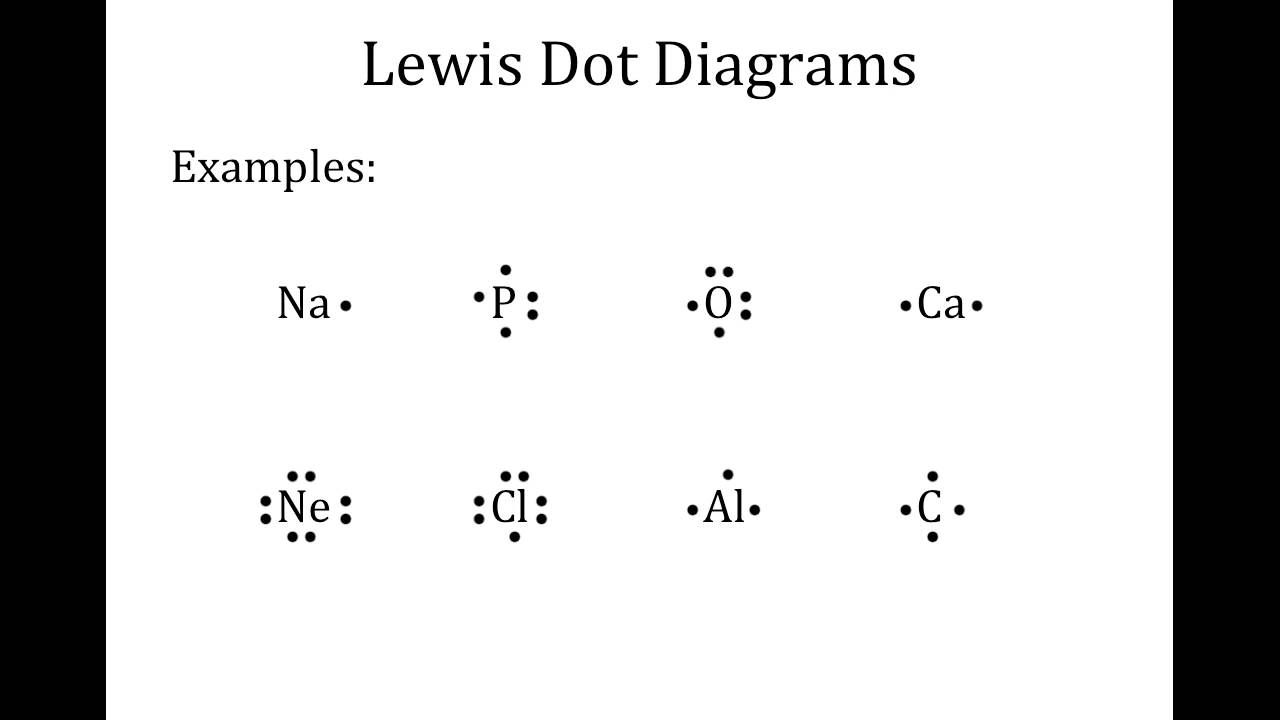
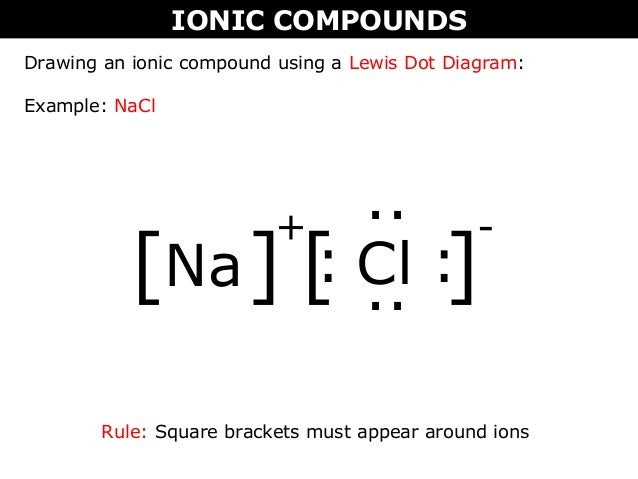
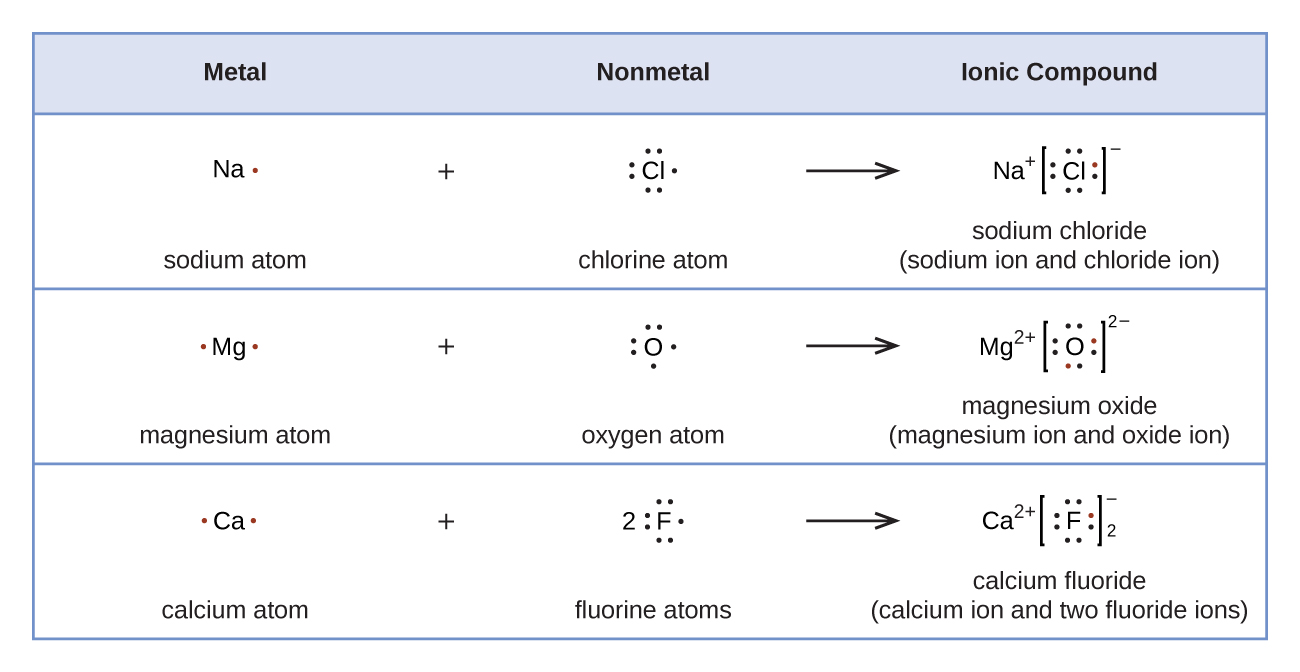
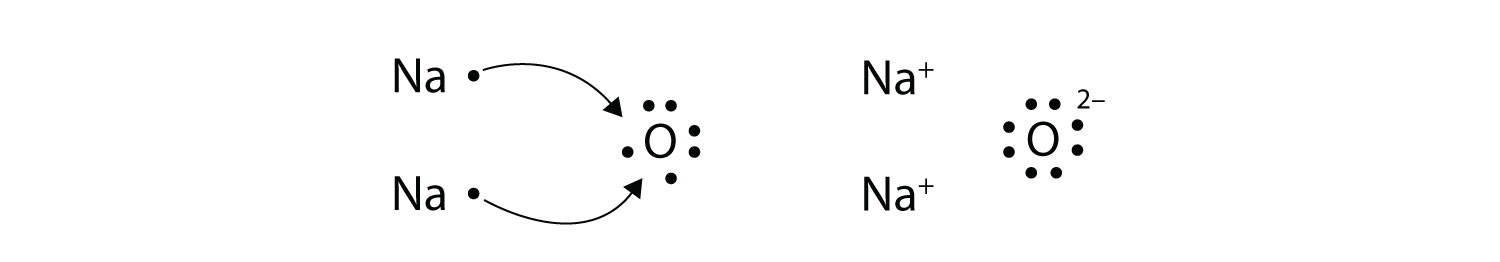
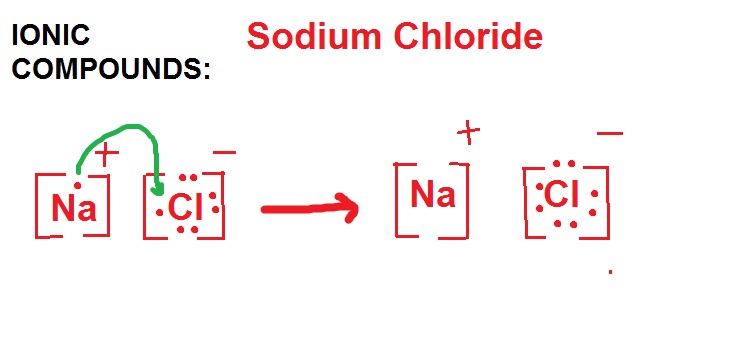
Lewi dot diagram ionic bond. We saw this in the formation of nacl. When you draw an ion dont forget and a charge. Diagrams that show electrons bonding and lone pairs of electrons. Lewis dot diagrams use dots arranged around the atomic symbol to represent the electrons in the outermost energy level of an atom.
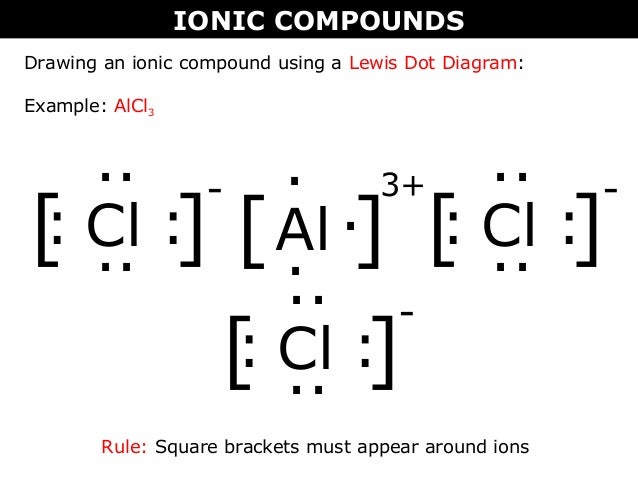
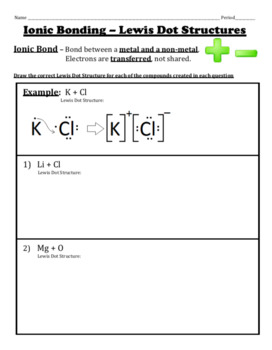
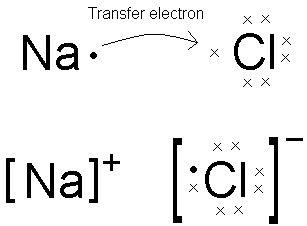
Lewis dot diagrams will continue to be useful throughout the unit and the semester. There are shorthand ways to represent how atoms form covalent or ionic bonds. A bond formed from the transfer of valence electrons from one atom to another. Atoms that gain or lose electrons forming a charge.
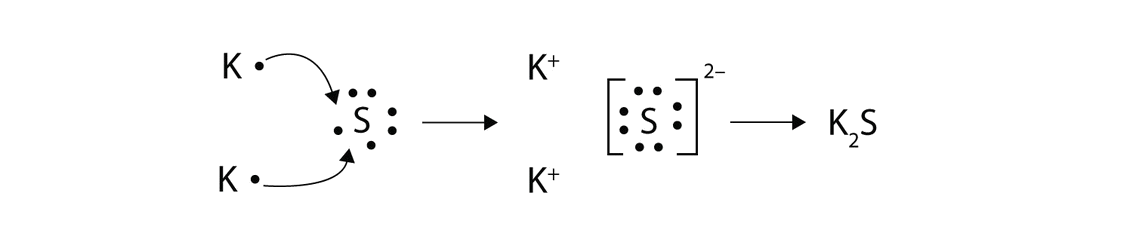
In both cases lewis dot structures were used to visualize the the bonding of the atoms in case of electron sharing or electron transfer. Chemistrylewisdotstructureakaelectrondotstructurenotes lewisdotstructuresforioniccompounds ioniccompoundsarecompoundsthatarecreated. Johannesson vsepr theory valence shell electron pair repulsion theory electron pairs orient themselves in order to minimize repulsive forces. In electron transfer the number of electrons lost must equal the number of electrons gained.
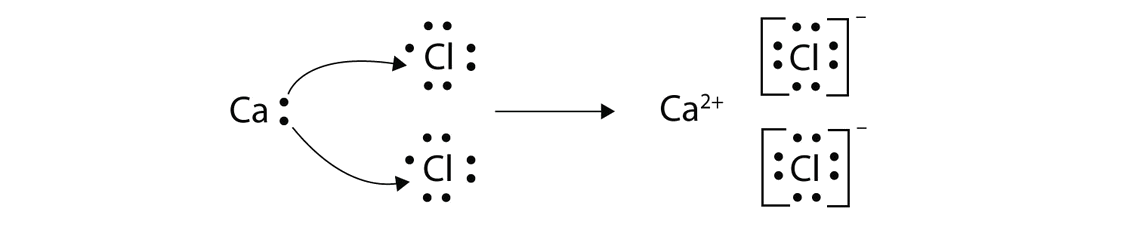
Ionic bonds are caused by electrons transferring from one atom to another. Ionic bonding in calcium chloride when calcium loses its two valence electrons to become an ion the lewis structure shows it with no dots electrons. The two ions attract each other according to coulombic interactions. Electrons on the outermost shell of an atom that participate in bonding.
Ionic lewis dot structures. As students are introduced to covalent bonding and continue to recognize periodicity in whether atoms gain lose or share electrons to form bonds. In an ionic bond one atom looses all its outer electrons leaving behind a filled inner shell while another atom gains electron s to fill its valence shell. The ca and cls are near each other but the two dots from each cl should not be interpreted as a covalent bond.