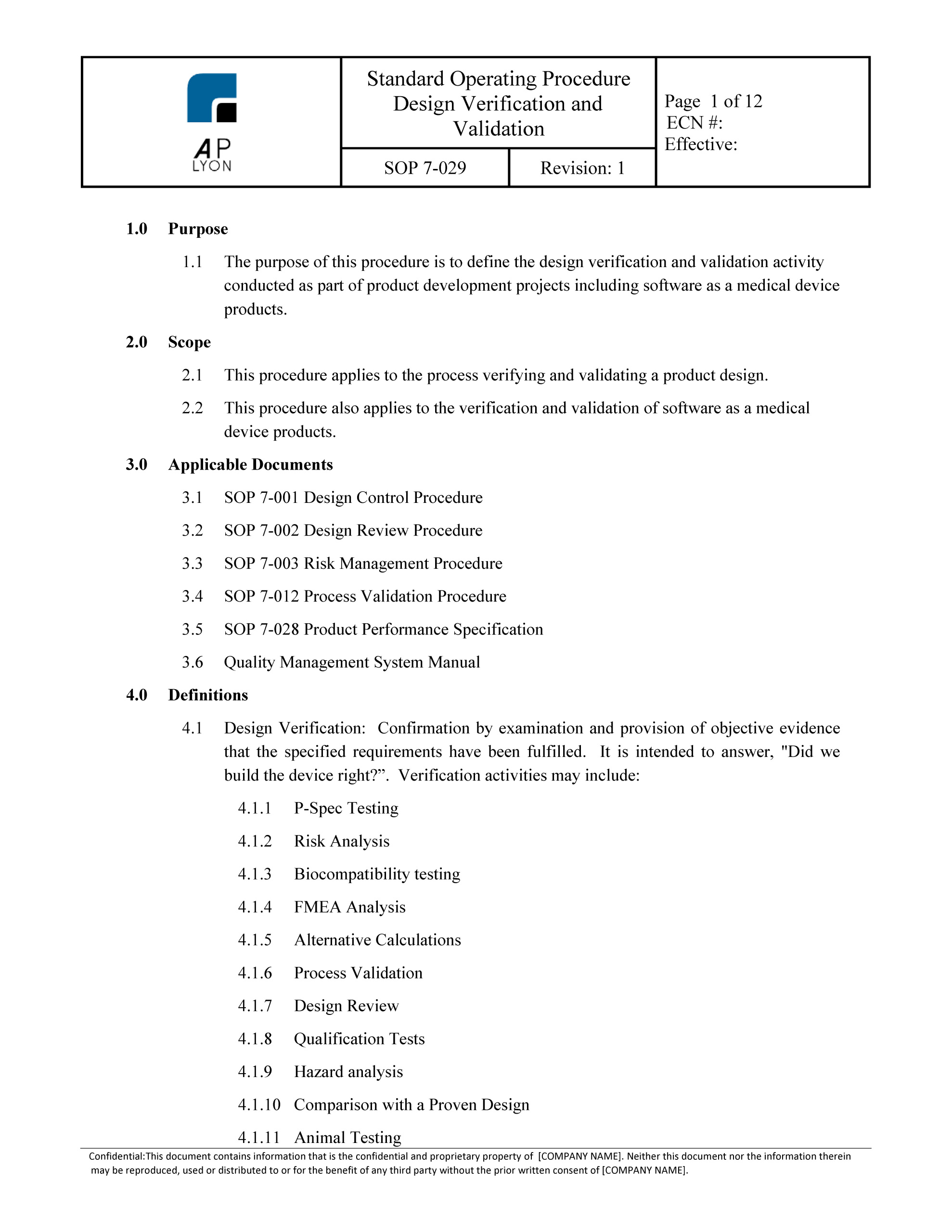
Medical Device Verification And Validation Plan Template

Month year revision sheet.
Medical device verification and validation plan template. We sat down with vv expert byron larson president of toltec ventures llc to discuss the latest trends in validation and verification. 1 41202 conversion to word 2000 format validation verification and. This article explains what a master validation plan is and explains when it is appropriate to have a master validation plan and when a master validation plan is unneeded. Im only focusing on these versions for the time being.
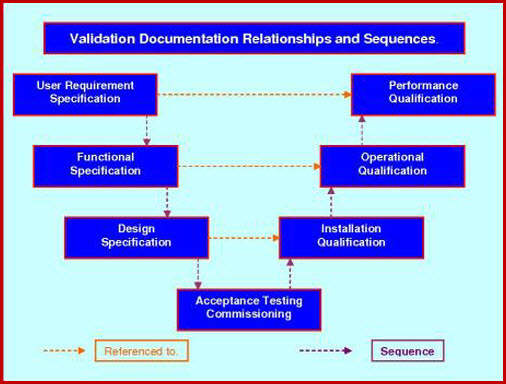
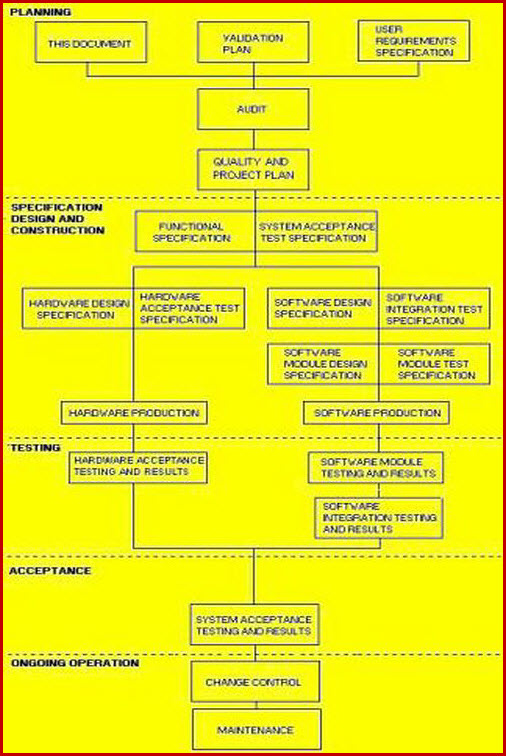
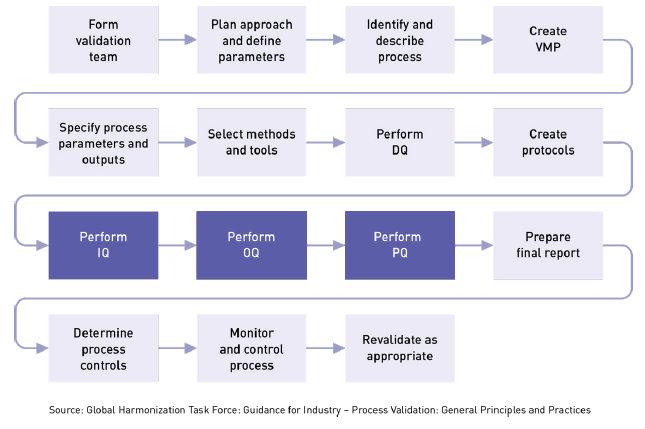
Date revision description rev. Heres how you handle verification and validation april 13 2018 by chris newmarker the two vs also known as vv verification and validation serve to link the medical device product that has been developed all the way back to the initial customer needs and product requirements. It should be a synopsis two to four pages in length of the major elements from all sections of the document with emphasis on vv scope ms requirements and acceptability criteria vv methodology and vv issues. 6 process validation in medical devices tuv sud validation master plan individual validation plan manufacturers often choose to develop a validation master plan vmp as a tool to control and monitor the status of validation activities.
Iso 13485 clause 752. The executive summary provides an overview of the vv plan. Department of housing and urban development. The focus of this post and the relevant terms for design controls are design verification and design validation.
As vmps are not required by regulations the content and structure can vary widely. At the same time the fda medical device templates business has become highly regulated. Today expect medical device templates validation to perform to a high level. Also to complicate matters a bit outside the medical device industry verification and validation also mean different things.
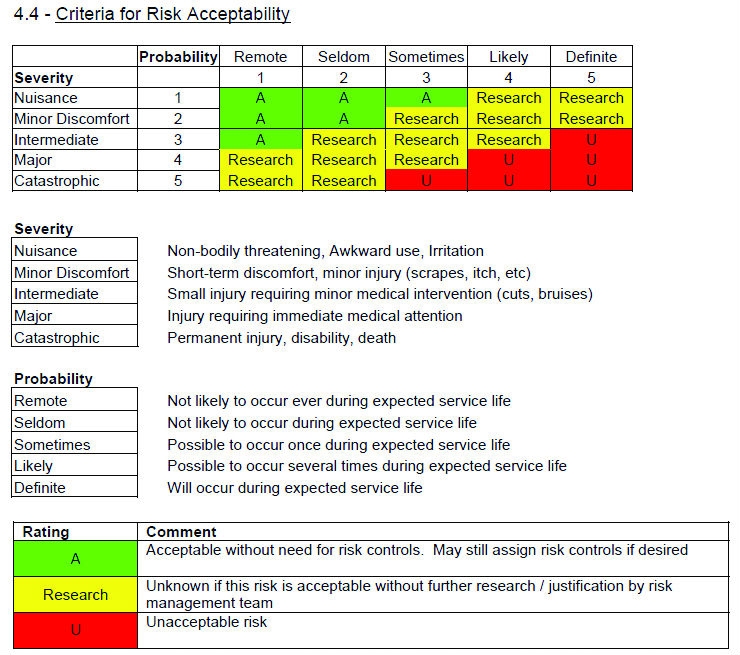
Project or system name. Aql sampling plans are not suitable for testing in the verification and validation phases. 0 53000 validation verification and testing plan template and checklist rev. In the united states there are two applicable regulations for medical device manufacturing process validation.
And each means something different. Toltec ventures helps medical device companies with all aspects of vv to improve production and comply with fdas qsr and isos 13485 quality system directives. The process of verification and validation is a critical component for the effective development and design of medical devices.