Pharmacovigilance Certificate Programs

Program applications are accepted until july 12.
Pharmacovigilance certificate programs. View the admissions checklist. We are pleased to announce that for the first time in canada aaps is offering the certificate in clinical safety and pharmacovigilance. Immediate access to training. These competencies outline the functional knowledge and skills needed to work in safety and pharmacovigilance and.
Pharmacovigilance a modernization and evolution of safety pharmacology employs sophisticated state of the art approaches in the collection and analyses of. Pharmacovigilance is the science of collecting detecting assessing and monitoring data of marketed drugs in order to minimize or prevent adverse effects. From the certificate in global pharmacovigilance. Benefit risk assessment towards their graduate degree provided they formally apply for admission to the ms program and are accepted by temple universitys graduate school.
Drug safety pharmacovigilance training apply for a training program or degree or certification course on qppv drug safety associate physician regulatory associate drug data analyst triage officer and auditor courses at sollers in edison nj. To receive the additional certificate in global pharmacovigilance. Application process the certificate in global pharmacovigilance. Attend classes in the evening and get the applied training you need to advance your career in this in demand profession.
Drug safety pharmacovigilance training apply for certification or degree on qppv drug safety associate physician regulatory associate drug data analyst triage officer auditor course at sollers in edison nj. Drug safety pharmacovigilance pharmacokinetics and pharmacodynamics training and professional certification programs. Instructions username password and receipt of payment emailed instantly upon online enrollment optimized for learning. Drug safety and pharmacovigilance certificate.
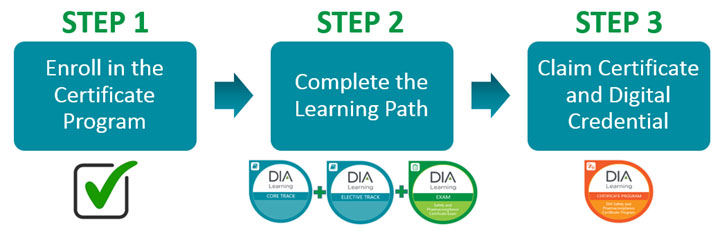
Dias safety and pharmacovigilance certificate program is a comprehensive program based on the dia safety and pharmacovigilance competency framework developed with experts working in the field. Use the button below to apply to durham tech following the health technologies enrollment steps then complete the medical product safety and pharmacovigilance program application available here february 1 2020. These competencies outline the functional knowledge and skills needed to work in safety and pharmacovigilance and comply with us and eu regulations. Dias safety and pharmacovigilance certificate program is a competency based program.
Online training the benefits of elearning. Students need to take five additional courses beyond the ms. This comprehensive program is based on the dia safety and pharmacovigilance competency framework developed with experts working in the field.