Source Documents For Clinical Trials Template

5 budgeting by activity worksheet this budget example from an uncomplicated pneumonia study is broken down by activities required at each study visit for each patient.
Source documents for clinical trials template. Welcome to global health trials tools and templates library. It is important to make clear to all study team members study monitors where applicable and potential auditors which document is the source the. Please note that this page has been updated for 2015 following a quality check and review of the templates and many new ones have been added. Source document plan guidance and template.
Signed protocol and amendments. Source document plan template version 1 january 2017. These templates are for pis and their study coordinators and should be used as examples or templates to build from and modify to meet their specific needs. As with all guidance documents they do not.
The forms serve only as templates. Source documents and regulatory binders october 6 2016 lisa wilson regulatory lead clinical trials office. Case report form crfsource document templates were created for university of wisconsin madison researchers. For example some investigators prefer to separate patient that participate in the trial from the regular patients and they like to have different form of source documents also some of them use different software for keeping medical records but when they participate in clinical trials they want to have paper source documents for patients.
Samples forms and worksheets compliments of mountainside md press and conducting clinical research. Guidance documents accessible from this page represent the agencys current thinking on good clinical practice gcp and the conduct of clinical trials. The most important purpose of source documentation in a clinical trial is to reconstruct the trial as it happened. These templates are consistent with the fda cdash clinical data acquisition standards harmonization standards.
Source documents in clinical trials. Protocol templates consent templates irb submission documents. Mock study section planning guide planning guide oncore clinical trial management system ctms new user request form required fields and definitions regulatory support and protocol templates find templates and forms on the institutional review boards irb website including. Anonymous commented in perfect clinical trial source documents.
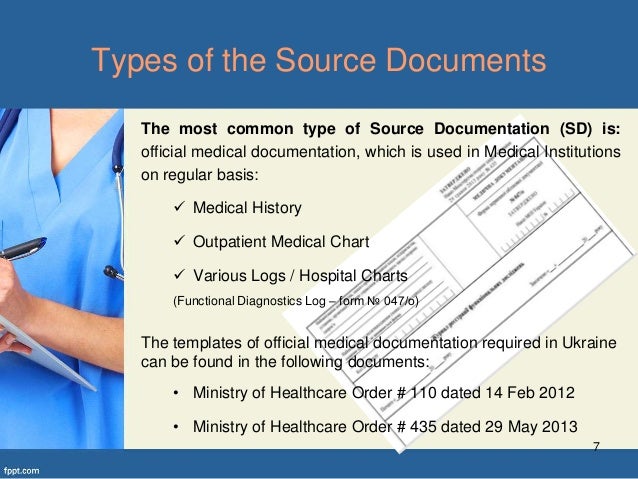
Hi some sponsors dont allow investigators to use source documents in clinical trials such as data worksheetstemplates arguing that source data should not contain instructions nor any other items logo etc which would not usually appear on a patient medical file. Clinical study management helpful templates.