Usp 800 Assessment Of Risk Template
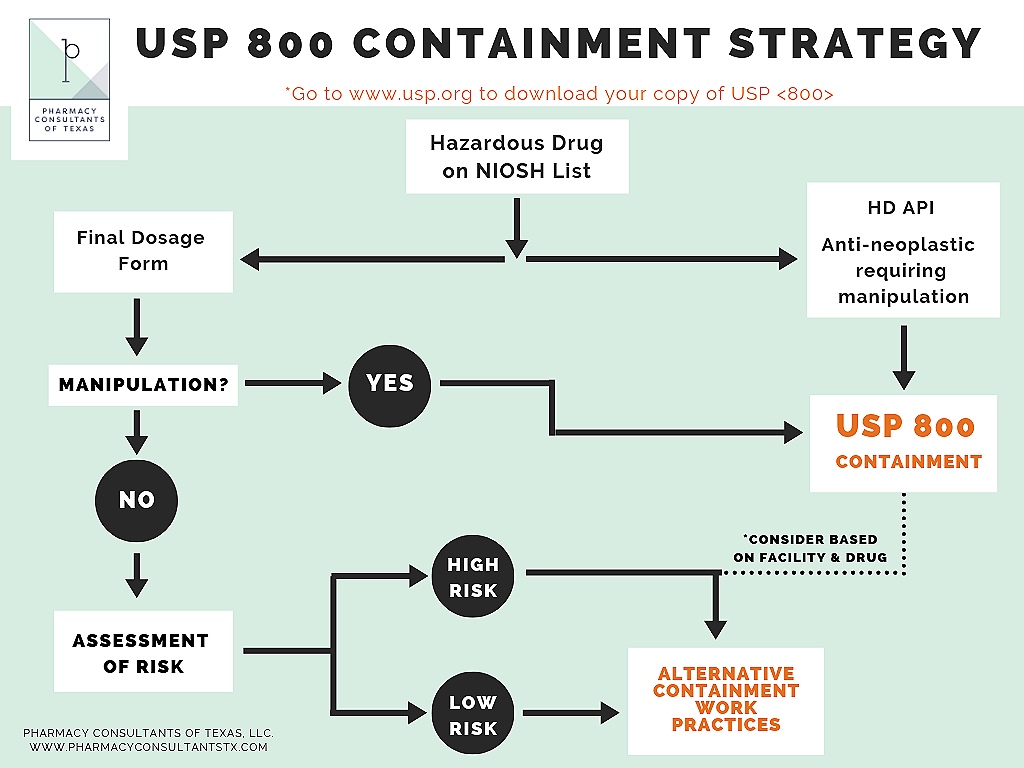
In the pursuit of usp 800 compliance the first step is to identify all of the hazardous drugs hds utilized by the entity as.
Usp 800 assessment of risk template. Perform an assessment of risk aor to determine which specific dosage forms of these agents may be handled with alternative containment strategies andor work practices. Handle in the same manner as apis and antineoplastic drugs that require manipulation using all of the containment properties and work practices listed in 800. Per usp 800 the assessment of risk aor allows for a pharmacy to provide alternative containment strategies. Pharmecology offers a unique assessment of risk customized to your inventory as required by usp 800 and also provides a one stop solution to help your organization identify segregate manage and dispose of pharmaceutical waste in a compliant and cost effective manner.
Portions of this information and these forms are proprietary to and subject to copyright ownership of clinical iq llc and have been modified by sample pharmacy under license and for limited use. Usp general chapter 800s implementation date is coming up on dec. A set of excel based risk assessment templates which can assist the user in this critical step of performing the risk assessment required by 800. Usp 800 assessment of risk once it has been determined which niosh hazardous drugs and dosage forms are in the pharmacy there are two options on how to handle the hazardous drugs.
As the hdcs is organized in parallel to the usp 800 preparation checklist users can rapidly refer to it for help completing the checklist. Some will require full containment others can be handled with gloves alone. Under usp 800 certain hazardous drugs on the niosh list must follow the chapters containment requirements unless an assessment of risk is performed and implemented. Under usp 800 certain hazardous drugs on the niosh list must follow the chapters containment requirements unless an assessment of risk is performed and implemented.
Usp general chapter 800s implementation date is coming up on dec. Sample pharmacy hazardous drug assessment of risk aor template. Use the containment strategies in usp 800 for all hazardous drugs or perform an assessment of risk. Ncpa has developed a blank usp 800 risk assessment template and a sample template for testosterone to help you create your own risk assessments for each hazardous drug as required by usp 800.
Cite the document that defines hazardous drugs identify the drugs and dosage forms eligible for an assessment of risk design an assessment of risk to be used at your organization list the facility and monitoring elements for compliance with usp 800 prioritize gaps in compliance that need to be addressed within your organization.