Mdr Essential Requirements Checklist Template
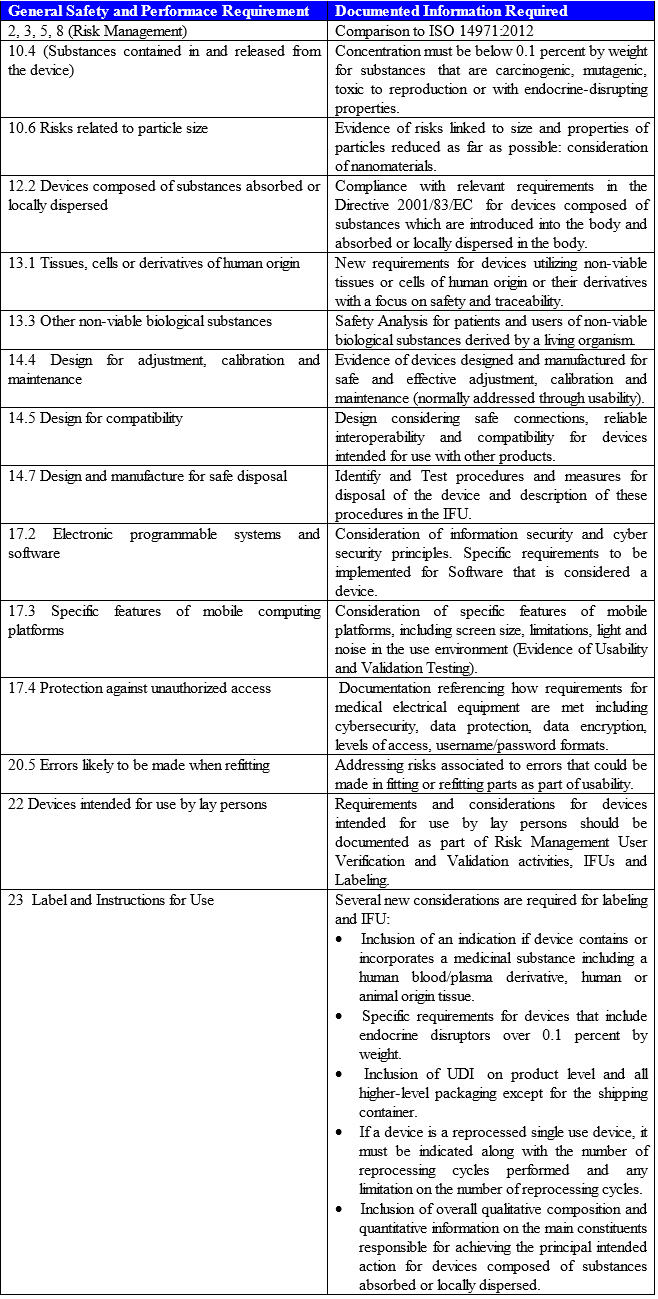
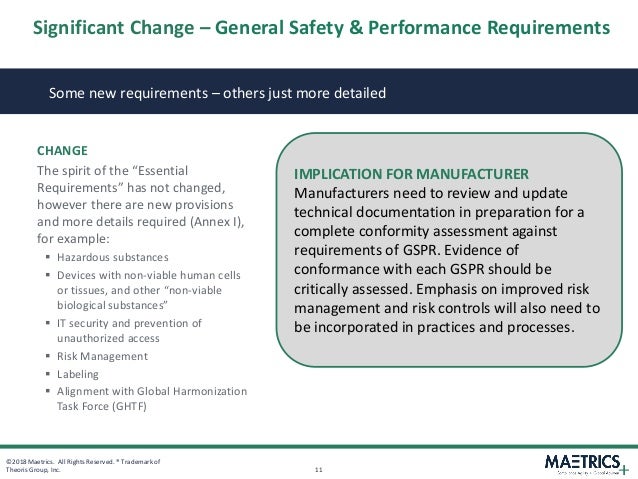
Those familiar the current mdds essential requirements covering thirteen areas and divided into two chapters will immediately see the similarities in the new eu mdr annex.
Mdr essential requirements checklist template. European medical device directive essential requirements checklist pdf160kb. Essential requirements ers are the requirements for safety and performance specified in annex i of the three medical device directives. These new requirements involve several changes that medical device companies must be prepared for including things like device classification and updating your qms. And most importantly the eu mdr has formalized the expectations that your qms documents records product information risk etc.
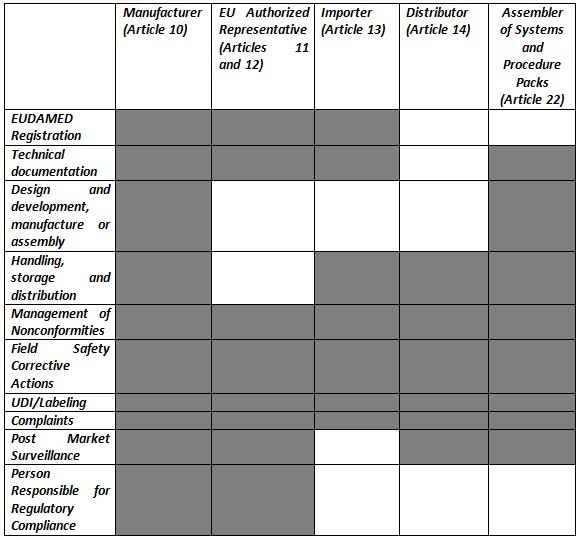
Requirements please refer to the corresponding articles. However the new annex i now contains requirements covering twenty two areas and is now divided into three chapters. Essential requirements annex i 9342eec as compliance the devices must achieve the performances intended by the manufacturer and be designed manufactured and packaged in such a way that they are suitable for one or more of the functions referred to in article 1 2 a as specified by the manufacturer. The essential requirements checklist is a important and crucial tool for manufacturers in the medical device industry to show compliance with the essential requirements of the european medical devices directives 9342ec 90385eec and 9879ec as outlined in annex i of all the directives.
Ers are divided into part i ie general requirements and part ii ie requirements for design and construction. Essential requirements checklist annex i of proposed eu regulations compromise amendment for medical device ce marking identity of the device and applicable configurationsvariants covered by this checklist. Checklist for exporters of medical devices from australia to the european community essential requirements annex i 9342eec as amended by directive 200747ec. A sample of the completed essential principles conformity checklist md ccl for a medical device to be listed the local responsible person with support from the manufacturer is responsible for demonstrating that the.